India’s COVID19 Vaccine: Nationalism, Symbolism, Realism
By
Oommen C. Kurian
| 6 Jan 2021
By Oommen C. Kurian | 6 Jan 2021
Despair was the leitmotif of 2020. Even countries with acclaimed health systems have been through a nightmare that is yet to end. In this gloomy, dismal scenario, as the world enters 2021, the multiple COVID-19 vaccines that have secured regulatory approvals across the world and are being rolled out to much fanfare, offer a glimmer of hope that the pandemic will at last be brought under control in the coming months. That hope is tinged with fear as a new mutant of the virus, which is far more infectious, is spreading in countries across continents, prompting its description as a ticking time bomb.
The global scramble for vaccines, with richer countries ensuring selective supply and stockpiling enough doses to vaccinate their populations many times over, while poorer countries, hat in hand, stand in the queue, has hit a crescendo. It is being called ‘vaccine nationalism’ by many commentators. Around 30 countries had begun vaccination programmes by 4 January, 2021, but none of them are from Asia or Africa, barring a handful of rich Middle-Eastern countries (Israel, Bahrain, Oman and Kuwait). India, the largest producer of vaccines for middle- and low-income countries, is the world’s insurance against the threats posed by ‘vaccine nationalism’.
As India awaits its vaccine rollout in a week or so, there are concerns being expressed by the scientific community about the integrity of the regulatory processes which have simultaneously offered conditional approval for restricted use of two vaccines, Covishield and Covaxin. Even medical practitioners desperate for the arrival of a vaccine, have expressed doubts about the difficulties they may have to face in convincing the public if a vaccine with no scientific data on efficacy is being administered as part of a national vaccination drive. Despite high level officials and the Health Minister, Dr Harsh Vardhan, clarifying that, as of now, Covishield is the mainstay of the rollout and Covaxin is approved as an additional weapon against potential mutant strains, a huge controversy has erupted.
The health ministry’s initial budget for the nation-wide vaccination drive is reportedly Rs. 50,000 crore. Just to put the amount in perspective, according to latest available data (2017-18 BE), government spending on health across ministries and departments, between the Centre and the States, is about Rs 2,00,000 crore. Given this fast-changing context, this commentary tries to answer six key questions about India’s COVID19 vaccination rollout, arguably the largest single health sector initiative in the country ever, with the help of available information.
How Safe and Effective are These Vaccines?
Both Covishield, manufactured by Serum Institute; and Covaxin, by Bharat Biotech; are proven to be relatively safe vaccines without major adverse reactions. The former, a double-dose vaccine currently under clinical trials in India, has demonstrated an efficacy of 62 percent from its global trials. The company has recently said that if a three-month gap between doses is maintained, the vaccine’s efficacy shoots up to 90 percent. However, there is no data as of yet to support this. Bharat Biotech has also made similar claims about Covaxin’s efficacy being at least 60 percent.
The fact remains that till date, no efficacy data has been made available by the producers of either of these vaccines being tested in India. Covishield, to its credit, has data to show that an equivalent vaccine tested elsewhere has shown efficacy ranging from 62 percent to 90 percent at different doses. At the same time, Covaxin’s claims on efficacy, as of now, remain purely speculative, despite its relatively good safety and immunogenicity profile. Covishield’s use is supported by the principle of regulatory reliance that the World Health Organisation (WHO) published in June 2020, advising regulators to partly rely on robust processes of other national regulators, where necessary.
Is the Indian Regulator Justified?
Over the past months of the pandemic, the Central Drugs Standard Control Organisation (CDSCO) of the Government of India has been criticised intermittently for being “too slow” as well as “too fast”. In any case, unlike countries like the UK, currently under severe attack by a new and more virulent strain of the corona virus, COVID-19 seems to be on a downswing in India. The urgent need to have a “backup vaccine” for the coming few weeks, as is being claimed by officials, does not exist.
Some may point to China and Russia, who approved vaccines early on without releasing efficacy data, to justify the Indian regulator’s actions. At the same time, it is important to note that China or Russia didn’t gain much in a public health sense from early vaccine approvals, as both countries have been hesitant to administer largely untested vaccines to their own populations. This is clear from the fact that till the new year, both countries had vaccinated only under 0.5% of their respective populations, in spite of having multiple early vaccine approvals in place by mid-2020.
However, as a major vaccine exporter, India has a lot to lose from acting on a purely symbolic impulse to show a ‘purely’ indigenous vaccine on the victory stand, undermining the scientific regulatory process. This is particularly important as unlike medicines, vaccines are given to healthy individuals and the success of a vaccine heavily depends on the public perception of it. Potentially, India can now be bracketed with Russia and China into an arbitrary BRICS-like category of regulatory laxity reflected by indigenous vaccine approvals without efficacy data. China, a major competitor to Indian vaccines in the global market, is trying to overcome such doubts by conducting overseas trials of its vaccines that got advance approvals domestically, but with very limited success.
The hasty approval of Covaxin to project a symbolic victory while short-circuiting regulatory integrity would have only harmed the long-term interests of Bharat Biotech and India, given that no overseas trials are taking place for Covaxin. Doubly so because approvals could have been attained through proper channels with only a few weeks’ wait. There is a history of Indian regulators cutting corners for drug approvals during the pandemic. But a global perception of lack of regulatory robustness couldn’t have come at a worse time, when the world needs Indian vaccines the most, owing to the ‘vaccine nationalism’ of the rich countries.
Do We Have Enough Vaccine Doses to Cover 300 Million People?
The global vaccine rollout has been on for weeks now, and has been much slower than anticipated, with only 12 million shots administered across 30 countries by 4 January 2021. The initial aim was to vaccinate 20 million people by December in the USA alone. India’s effort to vaccinate 300 million of a prioritised population—involving 600 million doses of Covishield and Covaxin—by August 2021 is Phase-1 of the country’s overall vaccination drive. Because of the strong manufacturing capacity of Indian companies like Serum Institute and Bharat Biotech, securing doses for this Phase-1 should not be a problem for the government.
The Serum Institute has almost 100 million doses ready and can reportedly manufacture 50-60 million doses of Covishield a month, potentially ramping up capacity to 100 million doses from March. Bharat Biotech, on the other hand, can reportedly produce 300 million a year and a substantial capacity expansion to 700 million doses is underway. In addition, vaccines like Sputnik, Novavax, and Zycov-D, which have ongoing clinical trials in India, have the capacity to produce hundreds of millions of doses through manufacturing facilities in the country.
For these reasons, finding vaccines for India’s Phase-1 won’t be a bottleneck if the government spreads the rollout across the coming months with more vaccine approvals along the way, despite these companies having international and private market commitments. However, if India wants to complete the rollout quickly, securing the required vaccines may be a problem. Potentially, India’s planned rollout can be over in just two months if each of its 29,000 vaccination centres cover around 200 people a day on average.
How Are Indian Vaccine Trials Doing?
As of now, a range of vaccines like Covishield by Serum Institute of India and AstraZeneca; Covaxin by Bharat Biotech and ICMR; ZyCoV-D by Zydus Cadila and Department of Biotechnology; Sputnik V by Dr Reddy’s Lab and Gamaleya National Centre, Russia; a vaccine by Biological E Ltd Hyderabad and MIT; a vaccine by Gennova Biopharmaceuticals, Pune, and HDT Biotech Corporation, USA; Novavax and other vaccine candidates from the Serum Institute are at different phases of trials in India. Most of these trials are conducted by manufacturers, and others have manufacturing contracts with Indian companies. In addition, Bharat Biotech is testing intranasal COVID-19 vaccine candidates and the Serum Institute is conducting overseas trials with international partners.
When are We Starting the Rollout and Who are Getting the Vaccines?
According to various officials, the national rollout could start from mid-January. Private manufactures like Serum Institute and Bharat Biotech, who have global supply commitments, are awaiting final regulatory approvals, export approvals as well as a go-ahead from global bureaucracies like the WHO. Manufacturers of leading vaccines in India have been clearly instructed by the Government of India to cater only to the national effort and not to private or export markets for now.
According to the guidelines published by the government, the COVID-19 vaccine will be introduced in a phased manner, with the first phase focusing on healthcare workers, frontline workers and the population at higher risk. The prioritisation process of groups, according to the document (Figure 1), will also depend upon the disease incidence and prevailing pandemic situation.
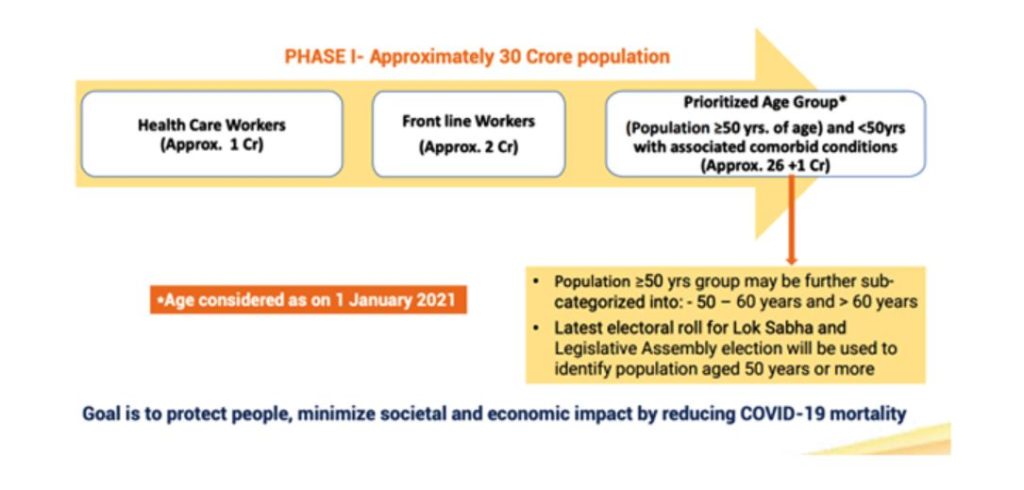
Figure 1: Phase-1 of COVID-19 Vaccine Introduction (source)
Will India Have Universal Vaccination?
Unfortunately, most of the benefits of the non-pharmaceutical interventions, like lockdown in India, have accrued to the relatively better off population groups, judging from findings of seroprevalence surveys which revealed very high seropositivity in slum areas across cities. It is likely that many parts of India have already achieved herd immunity through infection and the accompanying avoidable human cost. For the same reason, if the initial vaccination of 300 million most vulnerable citizens brings mortality by COVID-19 close to zero in most states, the government may rethink any existing strategy of a public-financed national level universal COVID-19 vaccination drive. If India, indeed, opts for universal vaccination against COVID-19, substantial investments may need to be made in improving cold-chain facilities, given the state-level variance that exists in the country (Figure 2)
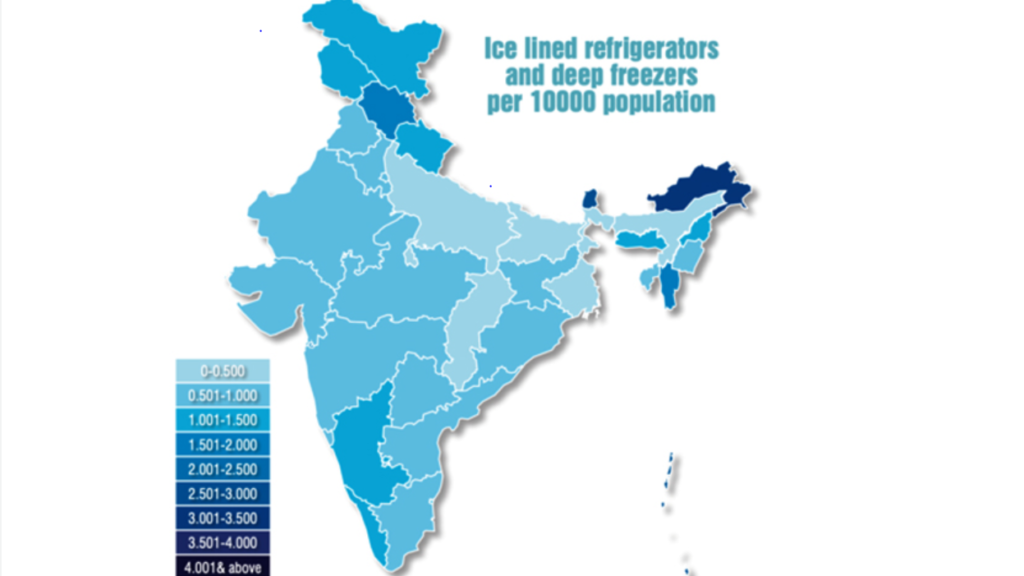
Figure 2: Ice lined refrigerators and deep freezers per 10000 population across Indian States (source)
Also, once the vaccination drive starts showing results on mortality, the number of COVID-19 tests across the country is bound to go down, as the current positivity rate is below 2%. This means, for detecting every positive case, currently around 50 test kits are used. The money spent on testing by the government is substantial, and each negative test in real terms means resources that could have helped immunise multiple people, or could have provided much needed care for many non-COVID conditions. In a severely resource-constrained health system such as India’s, the trade-offs between vaccination and testing and other interventions across the health system will be considered at some point and will dominate debates in this year. For the same reason, India may also consider introducing vaccines in the private market in the coming months as well.
The Way Forward
As we go forward with the vaccine rollout, which resembles a national election in scale, there may be multiple challenges which can affect the pace and trajectory of the initiative. First, with case numbers going down, many clinical trials in the country seem to be struggling to find participants. It is likely that the illusion of impending vaccine availability—aided by constant coverage in the media—may be impacting volunteers. Many seem oblivious to the possibility that big pharma companies like Pfizer and Moderna may not be making their vaccines available in the Indian market in the near future.
As thousands of master trainers are getting readied for the upcoming rollout, the Government of India on 30 December 2020 released an elaborate set of Covid-19 vaccine communication guidelines, which was welcomed by the scientific community as well-designed. However, it has been downhill since then, starting with the Subject Expert Committee’s (SEC) recommendation for emergency approvals which pointed to a distinction between Covishield and Covaxin, followed by the DCGI’s confusing approval which seemed to treat both vaccines at par.
In a context where political leaders are fanning vaccine hesitancy, it will be a struggle for health workers and vaccinators, and even political and community leaders, to build popular confidence in vaccines when key communication from the top fails due to hazy messaging. When we add this to the potential erosion of global trust in India’s governance structures, and Indian vaccines itself, one has to emphasise that by rushing the final phase of a scientific process to fulfil a largely symbolic urge, India has undermined its own role as a strong counterbalance to the rich countries’ vaccine nationalism.
Surely India does not want to go the way of China where many COVID-19 vaccines exist, but trust doesn’t?
The article first appeared in ORF
Disclaimer: Views expressed in the blog are the author's own
